Effects of Infant Formula with HMOs on Growth and Morbidity – Promising Trial Results

Human milk contains an abundance of structurally diverse oligosaccharides, known collectively as human milk oligosaccharides (HMOs), which represent the third largest solid component of human milk after lactose and lipids. Compare to human milk, cow’s milk contains a relatively low oligosaccharide content with limited structural diversity.
Emerging research has suggested that HMOs are extremely important in enhancing the development of the intestinal microbiota of a newborn, as well as supporting immune protection in breastfed infants. Therefore, HMOs represent a vital component of infant nutrition. Numerous studies in the past have concluded that breastfed infants have had a lower incidence of infections, including respiratory tract infections, compared with those fed infant formula.
While the safety and effects of HMOs as a possible ingredient of an infant formula has been established in preclinical studies, clinical data regarding the safety and effects of HMO supplementation on infant growth and tolerability have been limited up to now.
A recent clinical trial conducted jointly between paediatric teams at universities in Palermo, Italy and Hasselt, Belgium set out to evaluate infants fed a cow’s-milk-based formula supplemented with two HMOs. The two HMOs added to the formula were 2’fucosyllactose (2’FL) and lacto-N-neotetraose (LNnT), which are two out of the 10 most abundant oligosaccharides found in human milk. The primary objective of the study was to evaluate the growth of infants fed the HMO-supplemented formula. The secondary objective included the evaluation of anthropometric measures, GI tolerance and behavioral patterns, as well as morbidity through age 12 months.
175 term healthy formula-fed infants were enrolled in the trials, The groups were then randomized equally with half receiving the Control intact protein, cow’s–milk-based whey-predominant infant formula and the other half receiving the same formula but with added HMOs (the Test formula). The infants were fed their assigned formula up to six months of age, after which all infants received standard follow-up formula without HMOs from 6-12 months Complementary foods were allowed beginning at four months of age. Clinical examinations of the infants were carried out at 14 days and followed by visits at 1,2,3,4,6, and at 12 months of age.
The study demonstrated comparable weight gain in the Test and Control groups and growth according to the WHO growth standards through 12 months of age. The study also showed that the Test formula supplemented with HMOs was well tolerated.
According to parental reports, infants fed the Test formula had significantly softer stools, less episodes of “woke up at night” at 2 months of age and less colic in subgroup of cesarean-born infants at age of 4 months. Collectively, these findings suggest that infant formula supplemented with HMOs may confer improved GI comfort, although further studies are needed.
There were a similar number of total reported AEs in the Test and Control groups, which demonstrates the safety of the Test formula. Importantly, the study observed significantly lower rates of bronchitis and lower respiratory-tract-related morbidity outcomes through 12 months among subset of cesarean-born infants fed the HMO-supplemented formula, compared to those fed the Control formula. There was a lower likelihood of reporting of the use of antipyretic which was significant at 4 months of age and antibiotic medications through 12 months in the Test group.
It is also important to note that most of the morbidity-related differences between the Test and Control groups persisted through 12 months, although the HMO-supplemented formula was stopped at six months. The potential immune-modulation of HMOs may be long-lasting: early exposure to HMOs may programme the immune system in a way that lowers the later risk of infections within the respiratory tract.
A limitation of the study is that there is no comparable data from a breast-fed reference group in terms of growth and the secondary outcomes.
While additional studies are needed to confirm whether HMO-supplemented infant formulas confer protection from illness, if the results of this recent trial are replicated the effects of HMOs on morbidity and medication use will carry substantial positive implications for the health of infants.
Overall, the trial demonstrated that infant formula supplemented with 2 HMOs (2’FL and LNnT) is safe, well-tolerated and supports age appropriate growth. Secondary outcome findings showed associations between HMO-supplemented formula and lower parent-reported morbidity (particularly bronchitis) and medication use (antipyretics and antibiotics).
If you liked this post you may also like
Disorder of Gut-Brain Interaction: Insights, Causes and Management
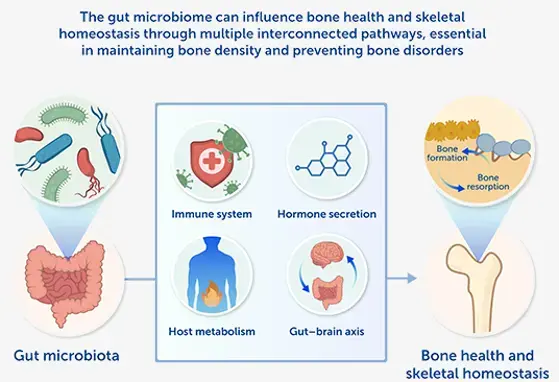
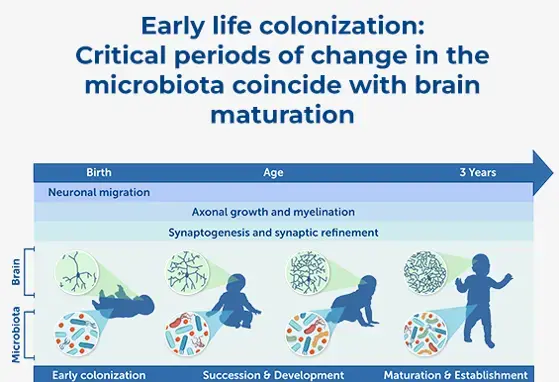
Microbiome and Brain Development: A Tale of Two Systems
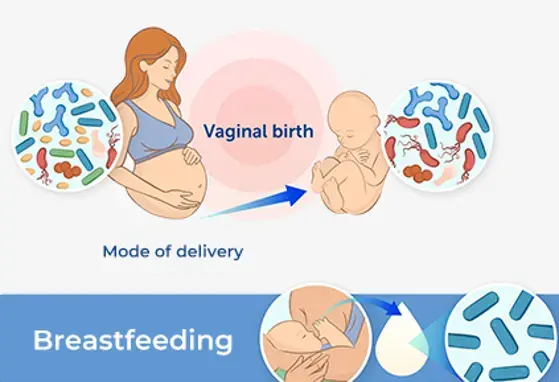
An Overview of Early-Life Gut Microbiota Modulation Strategies